[最も選択された] yield formula chem 219222-Percent yield formula chemistry
Calculating theoretical and percent yield You learned how to calculate theoretical yield and percent yield in general chemistry lab Since chemistry is a cumulative discipline, we expect students to remember topics from previous chemistry course Anyway, here is a brief recap write the balanced chemical equation of the reactionScience AP®︎/College Chemistry beta Chemical reactions Stoichiometry Stoichiometry Stoichiometry Worked example Calculating amounts of reactants and products Limiting reactant and reaction yields This is the currently selected itemThe chemical equation shows iron(III) phosphate reacting with sodium sulfate 2FePO4 3Na2SO4 >Fe2(SO4)3 2Na3PO4 What is the theoretical yield of Fe2(SO4)3 if 00 g of FePO4 reacts with an excess of Na2SO4?
Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau
Percent yield formula chemistry
Percent yield formula chemistry-Multiply the answer that you got in Step 4 by 100 to get your final actual yield This actual yield is expressed as a percentage of the theoretical yield;So, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World
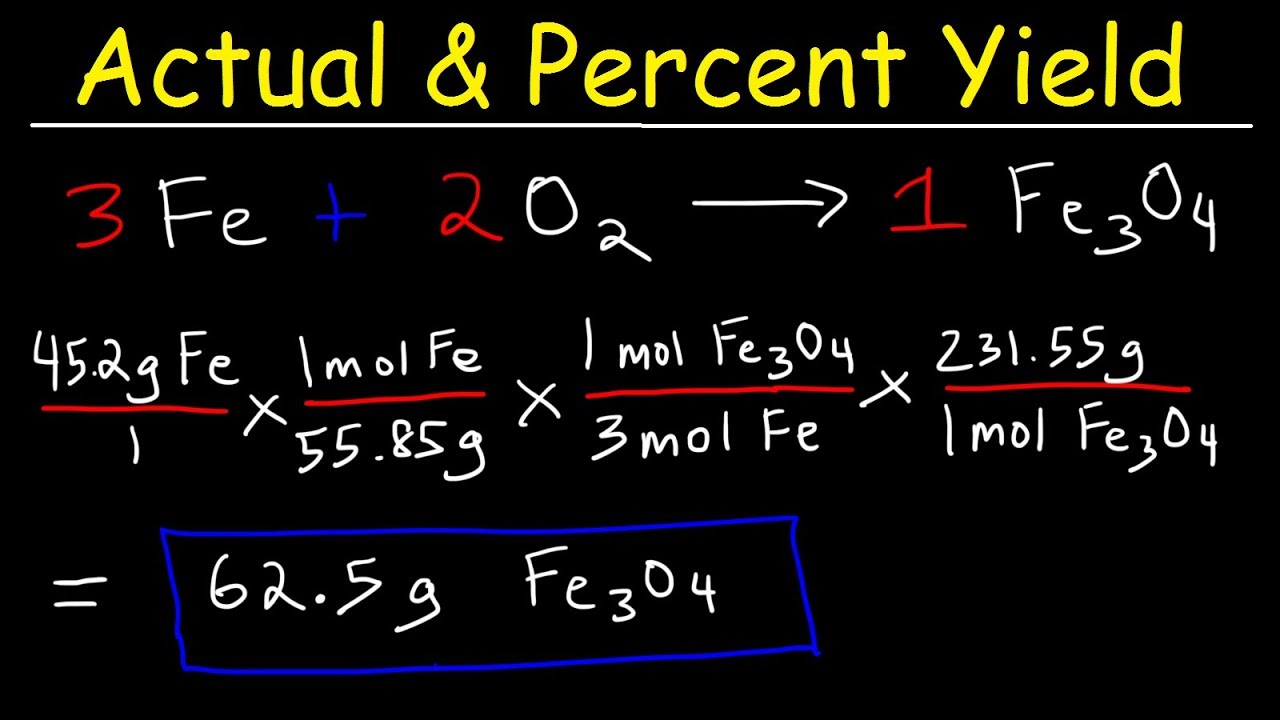
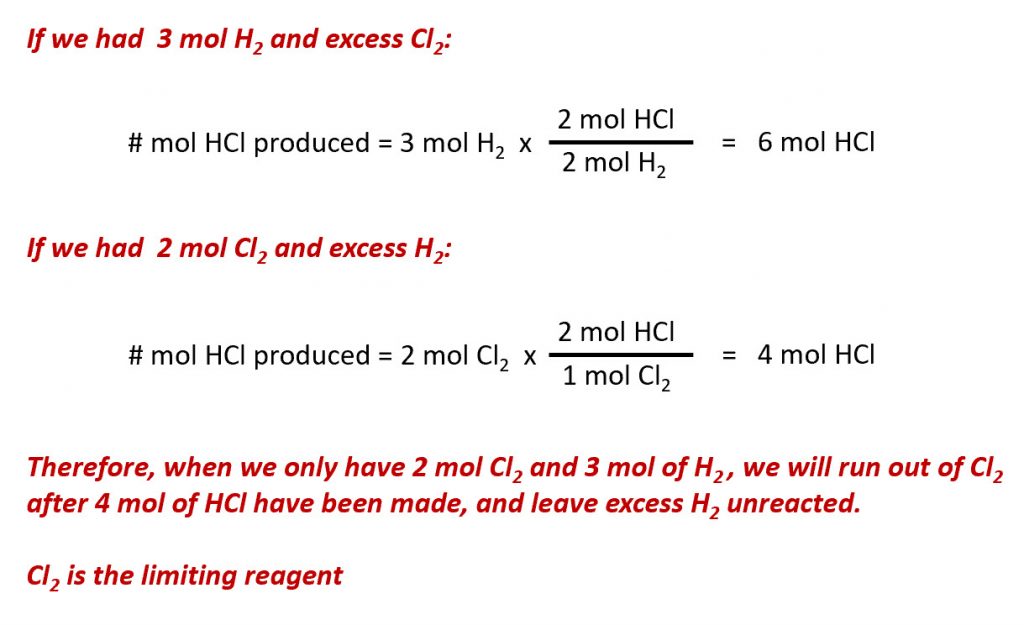
To determine the theoretical yield of any chemical reaction, multiply the number of moles by the molecular weight Theoretical yield will be calculated in grams because it uses the theoretical yield equation and it is the amount of the expected product Now we will solve example with theoretical yield formula to make it more clearThe reactant yielding the lesser amount of product is the limiting reactant For the example in the previous paragraph, complete reaction of the hydrogen would yield mol HCl produced = 3 mol H2 × 2 mol HCl 1 mol H2 = 6 mol HCl mol HCl produced = 3 mol H 2 × 2 mol HCl 1 mol H 2 = 6 mol HClC7H16 11O2 > 7CO2 8H2O Step One Identify the limiting reagent (the question only gives you one of the reactants, therefore, making that compound the limiting reagent) The limiting reagent is O2 Step Two Find the theoretical yield
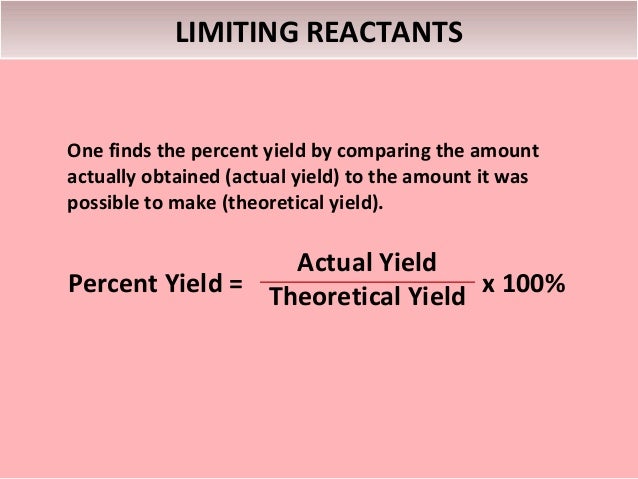
The ratio of carbon dioxide to glucose is 61 You expect to create six times as many moles of carbon dioxide as you have of glucose to begin with The theoretical yield of carbon dioxide is (0139 moles glucose) x (6 moles carbon dioxide / mole glucose) = 04 moles carbon dioxidePractice some actual yield and percentage problems below 1 For the balanced equation shown below, if the reaction of 408 grams of C6H6O3 produces a 390% yield, how many grams of H2O would be produced ?Percent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%
Current Yield = (Price Increase Dividend Paid) / Current Price In the above example, the current yield comes to ($ $2) / $1 = 013, or 13% When a company's stock price increasesBecause 28 is 1/th of 56, so theoretically you can get 1/th of g of FeS or 44g 2nd the % yield calculation itself % yield = actual amount obtained x 100 / maximum theoretical amount possible % yield = 41 x 100 / 44 = 932% (to 1dp, 3sf) More examples of % yield and atom economy calculations in section 66O 2 C 6 H 12 O 6 6 → CO 2 6H 2 O After balancing the equation, now we know that after the actual reaction if there is no loss of reactants, our product will be six molecules of carbon dioxide and six molecules of water It is the theoretical yield However, it is impossible for a ration to give 100 percent yield



Chem Unit 10 Presentation



Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab
Still, if you want to do the calculation by hand, you should use the percent yield equation percent yield = (experimental mass of the desired product / theoretical mass of the desired product) * 100 For this equation, you must know two out of the three valuablesEmail Link Tweet Percent Yield Moderators Chem_Mod, Chem_Admin 6 posts • Page 1 of 1 Anjali_Kumar1F Posts 62 Joined Fri Sep 28, 18 725 am Percent Yield Post by Anjali_Kumar1F » Wed Oct 03, 18 351 amTotal yield (%) = (yield 1 st react × yield 2 nd react × , etc ) × 100 For example, in a chemical transformation composed of three reactions and with partial yields each of 25%, 50%, and 75%, respectively, to calculate the total yield the above equation is applied (expressing the partial yields to decimals), resulting the following expression



Solved Chem 30a Homework Week 10 Stoichiometry 6 Back T Chegg Com



Measurement Uncertainty Accuracy And Precision Chemistry 2e
Cyclohexene = 010 mol, Br 2 = 011 mol, dibromocyclohexane = 00 mol Limiting reagent is therefore the cyclohexene Theoretical yield of dibromocyclohexane is 010 mol, therefore, experimental yield = 00/010 = % © Dr Ian Hunt, Department of ChemistryBoard index Chem 14A Review of Chemical & Physical Principles Limiting Reactant Calculations;Yield}$$\times$ 100% The amount of product actually made compared with the maximum calculated yield is called the percentage yield



Process Chemistry Wikipedia


How To Calculate Percentage Yield In Chemistry How To Wiki
The theoretical yield refers to the amount that should be form when the limiting reagent is completely consumed The actual yield is expressed as a percentage of the theoretical yield This is called the percent yield To find the actual yield, simply multiply the percentage and theoretical yield togetherBoard index Chem 14A Review of Chemical & Physical Principles Limiting Reactant Calculations;If your actual yield is 76, then this means that you recovered 76 percent of the product that you would have gotten if your reaction were 100 percent efficient


Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities


Quantitative Chemistry Theoretical And Percent Yield
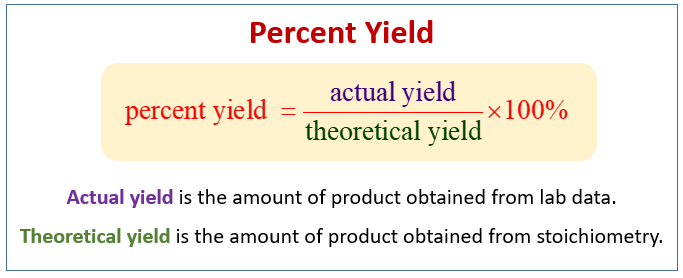
Percent Yield Formula The equation for percent yield is percent yield = (actual yield/theoretical yield) x 100% Where actual yield is the amount of product obtained from a chemical reaction theoretical yield is the amount of product obtained from the stoichiometric or balanced equation, using the limiting reactant to determine productTheoretical Yield Formula Solved Examples & Practice Questions In theory, we can always predict the amount of desired product that will be formed at the end of a chemical reaction Assuming that the reaction will go to completion we can predict this amount of product from the stoichiometric coefficients of the balanced chemical equationThe formula for percent yield is Example The medical drug aspirin is made from salicylic acid 1 mole of salicylic acid gives 1 mole of aspirin Given that the chemical formula for salicylic acid is C 7 H 6 O 3 and the chemical formula for aspirin is C 9 H 8 O 4


How To Calculate The Percent Yield And Theoretical Yield Youtube


Q Tbn And9gcsbwel4mzamey6ite9bp 08j Tpx0zptf7ruyvck4xodffyxfeu Usqp Cau
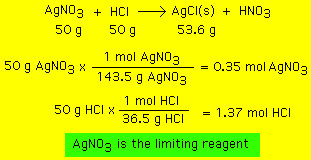
The question reads that the actual yield from 100 grams of Calcium Hydroxide is 50 grams of Calcium Oxide Hence, the percent yield is given by Percent Yield = Actual Yield/Theoretical Yield xCalculate the maximum theoretical yield of calcium oxide that can be produced from 250 g of calcium carbonate Write down the balanced chemical equation CaCO 3 \(\rightarrow\) CaO CO 2The yield calculation that you perform for most synthetic procedures is based on the comparision of moles of product isolated and moles of product that you can theoretically obtain based on the the limiting reagent For that let's look at one simple example A student reacts 350 g acetic acid and 100 mL of ethanol in the presence of 2 mL of


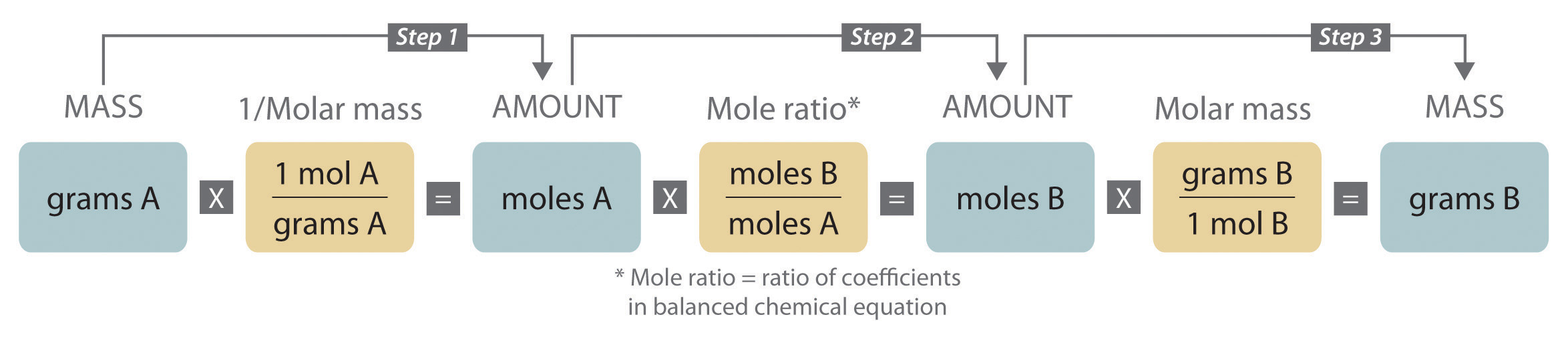
Ch150 Chapter 6 Quantities In Chemistry Chemistry



How To Find Actual Yield Theoretical Yield And Percent Yield Examples Practice Problems Youtube
6O 2 C 6 H 12 O 6 6 → CO 2 6H 2 O After balancing the equation, now we know that after the actual reaction if there is no loss of reactants, our product will be six molecules of carbon dioxide and six molecules of water It is the theoretical yield However, it is impossible for a ration to give 100 percent yieldChemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actualCalculating theoretical and percent yield You learned how to calculate theoretical yield and percent yield in general chemistry lab Since chemistry is a cumulative discipline, we expect students to remember topics from previous chemistry course Anyway, here is a brief recap write the balanced chemical equation of the reaction



Calculating Amounts Of Reactants And Products Worked Example Video Khan Academy


Chemical Reactions And Stoichiometry Chemistry Library Khan Academy
Measuring the amount of product formed gives us the actual yield From the ratio of the actual yield to the theoretical yield, we can calculate the percentage yield Percentage yield = $\frac{Actual\;Percent yield = purified percent yield = amount of P (g) theoretical yield (g) •100 Percent yield (if stoichiometry is 11) = amount of P (mol) amount of LR (mol) •100 Complicated balanced equations are uncommon in organic chemistry Many organic reactions have a stoichiometry of 11 in the balanced equationTheoretical Yield Formula The quantity of a product obtained from a reaction is expressed in terms of the yield of the reaction The amount of product predicted by stoichiometry is called the theoretical yield, whereas the amount obtained actually is called the actual yield A reaction yield is reported as the percentage of the theoretical amount



Reaction Stoichiometry Boundless Chemistry



Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube
Organic Chemistry FriedelCrafts Alkylation of Dimethoxybenzene Description & Background 0027 mol salicylic acid X g = 372g mol 3 Divide the number of grams of product obtained experimentally, by the number of grams obtained in the theoretical yield calculations and multiply by 100 to calculate the percent yieldYield is the mass of product formed in a chemical reaction Actual yield is the mass of product formed in an experiment or industrial process Theoretical yield is the mass of product predicted by the balanced chemical equation for the reaction Percentage yield = (actual yield ÷ theoretical yield) × 100 Optimum yield is the best possible yield achieved for a set of given reaction conditionsTo express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured) Steps



Yield Calculations Faculty Staff Sites


Mass Relationships In Chemical Equations
This video shows you how to calculate the theoretical and percent yield in chemistry The theoretical yield is the maximum amount of product that can be proPercent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%To calculate the theoretical yield, consider the reaction (2) CO ( g) 2 H 2 ( g) → CH 3 OH ( l) (3) 280 40 3 ( stoichiometric masses in g, kg, or tons) 12 t o n s H 2 × 3 C H 3 O H 40 H 2 = 96 t o n s C H 3 O H Thus, the theoretical yield from 12 metric tons (12x10 6 g) of hydrogen gas is 96 tons



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World



Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube
Email Link Tweet Percent Yield Moderators Chem_Mod, Chem_Admin 6 posts • Page 1 of 1 Anjali_Kumar1F Posts 62 Joined Fri Sep 28, 18 725 am Percent Yield Post by Anjali_Kumar1F » Wed Oct 03, 18 351 am% yield = actual amount of desired chemical obtained x 100 / maximum theoretical amount that could be formed If the reaction doesn't work the yield is zero or 0% If the reaction works perfectly and you obtain all the product, the yield is 100%, BUT this never happens in reality (as already discussed above)How to calculate the percent yield?
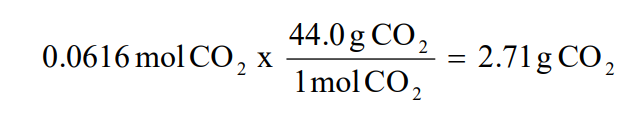


Molecular Formulas And Nomenclature


Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau
1) For the balanced equation shown below, if the reaction of 192 grams of O2 produced 676 grams of H2O, what is the percent yield?Theoretical Yield Formula Questions 1 Determine the theoretical yield of H 2 O (in moles) in the following reaction, if 25 moles of hydrogen peroxide are decomposed 2H 2 O 2 → 2H 2 O O 2 Answer In this reaction there is only one reactant (H 2 O 2) so it must be the limiting reactant Stoichiometry will be used to determine the moles of water that can be formed©21 Alison Frontier, University of Rochester Supported by a grant from the National Science Foundation NSF Funding {} This material is based upon work supported by the National Science Foundation under Grant Number CHE Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the
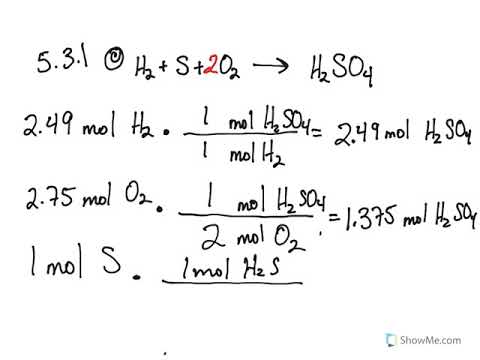


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



Reaction Percent Yield Introduction And Practice Exercises
The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage (1291) Percent Yield = Actual Yield Theoretical Yield × 100 % Percent yield is very important in the manufacture of products Much time and money is spent improving the percent yield for chemical productionHow to calculate the percent yield?The formula for percent yield is Example The medical drug aspirin is made from salicylic acid 1 mole of salicylic acid gives 1 mole of aspirin Given that the chemical formula for salicylic acid is C 7 H 6 O 3 and the chemical formula for aspirin is C 9 H 8 O 4



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
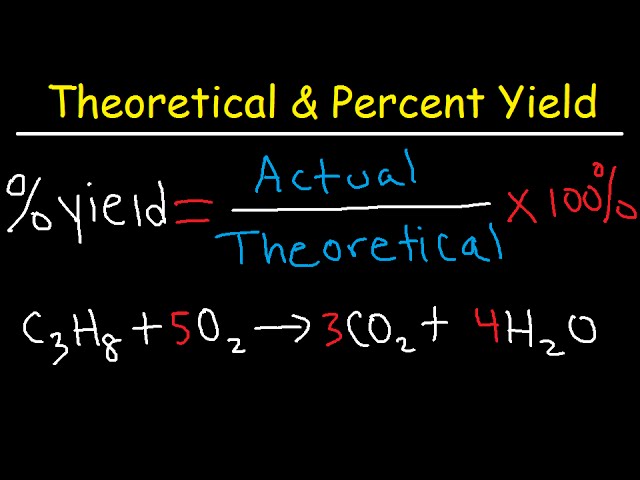


How To Calculate Theoretical Yield And Percent Yield Youtube
The absolute yield can be given as the weight in grams or in molar (molar yield) while the fractional yield or relative yield or percentage yield is calculated by dividing the amount of the obtained product in moles by the theoretical yield in moles The percentage yield is obtained by multiplying the fractional yield by 100%In chemistry, yield, also referred to as reaction yield, is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes In chemical reaction engineering, "yield", "conversionA chemist making geranyl formate uses 375 g of starting material and collects 417g of purified product Percentage yield is given as 941% Solution The actual yield is 417 g which is the quantity of the desired product Percentage yield is 941% Therefore, Theoretical yield=(Actual yield/percentage yield) x 100 = (417 / 941)100 = 443g


Calculation Of Theoretical Yield Organic Chemistry I 212 01
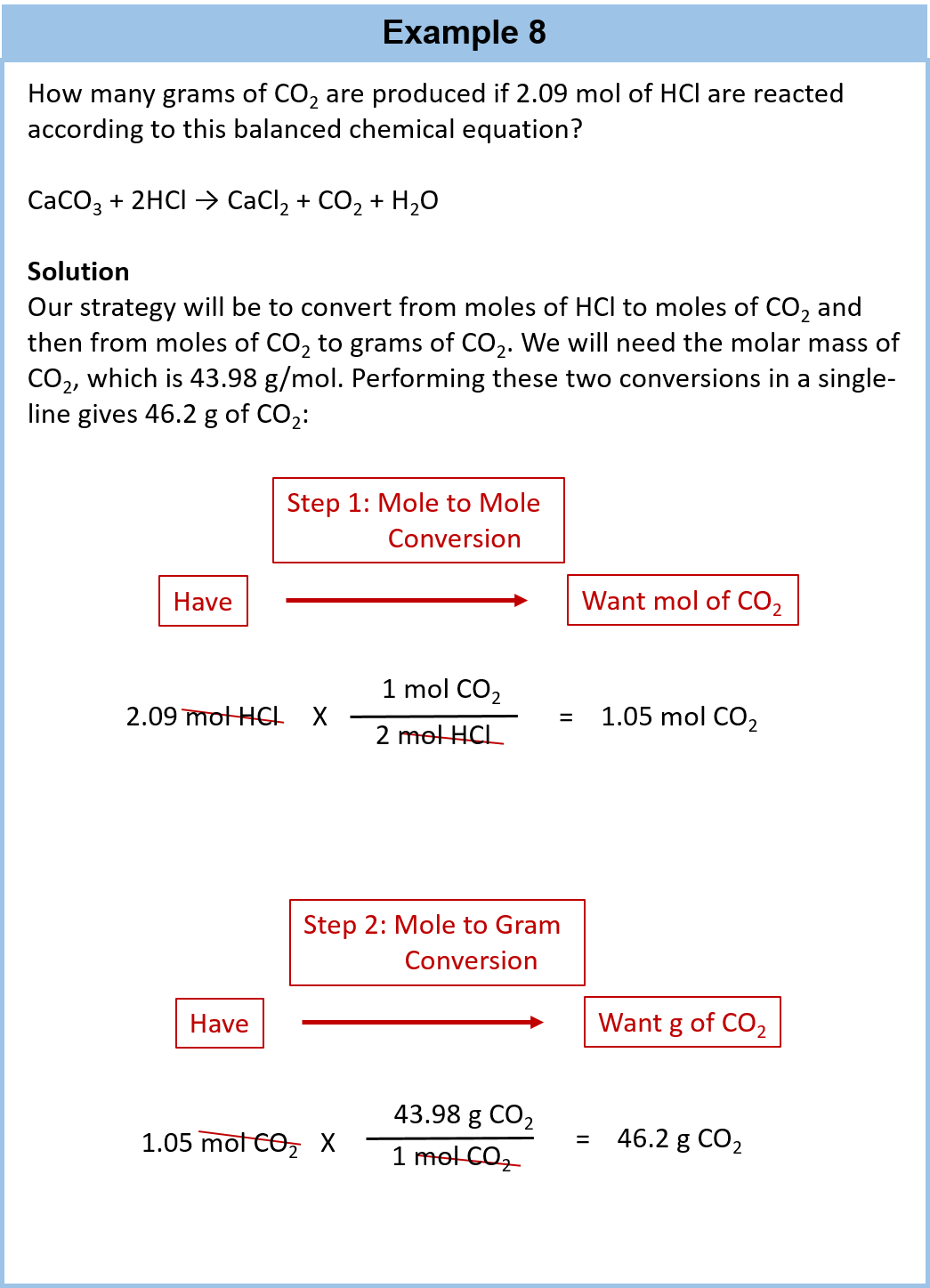


Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry
Before performing chemical reactions, it is helpful to know how much product will be produced with given quantities of reactants This is known as the theoretical yieldThis is a strategy to use when calculating the theoretical yield of a chemical reactionThe absolute yield can be given as the weight in grams or in molar (molar yield) while the fractional yield or relative yield or percentage yield is calculated by dividing the amount of the obtained product in moles by the theoretical yield in moles The percentage yield is obtained by multiplying the fractional yield by 100%The actual yield is the actual amount of product that is produced in a chemical reaction The theoretical yield refers to the amount that should be form when the limiting reagent is completely consumed The actual yield is expressed as a percentage of the theoretical yield This is called the percent yield To find the actual yield, simply multiply the percentage and theoretical yield together
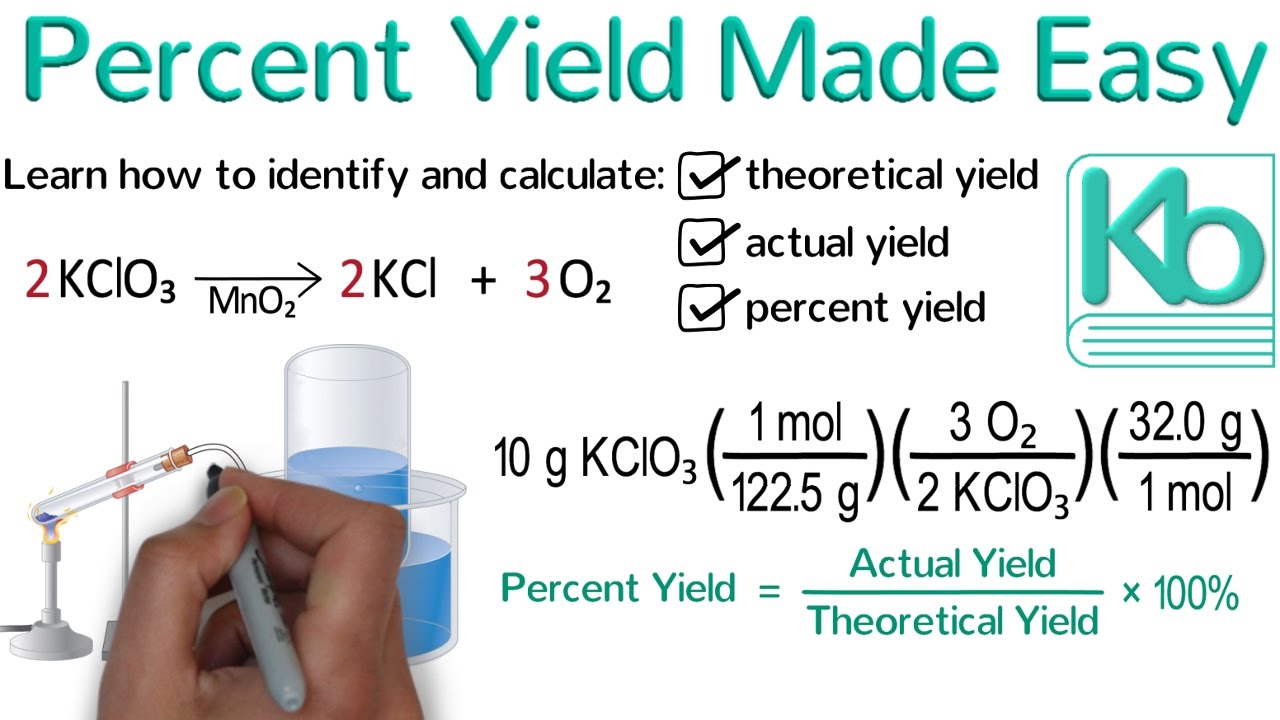


Percent Yield Made Easy Stoichiometry Tutorial Part 4 Youtube



Yields Introductory Chemistry
So, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)
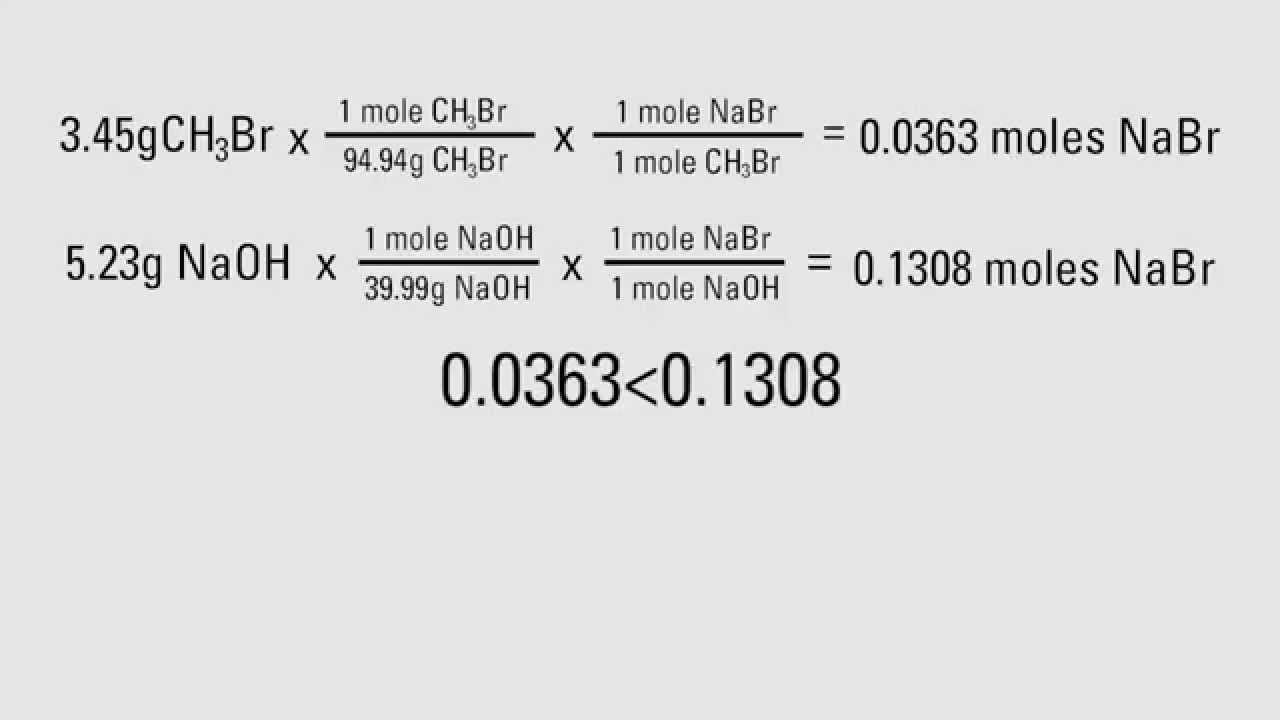


How To Calculate Theoretical Yields Youtube



How To S Wiki How To Calculate Percentage Yield



How To Calculate Percent Yield Science Chemistry Chemistry Class Chemistry



Percent Yield Chemistry Video Clutch Prep


3



Percentage Yield Lab Answers Schoolworkhelper
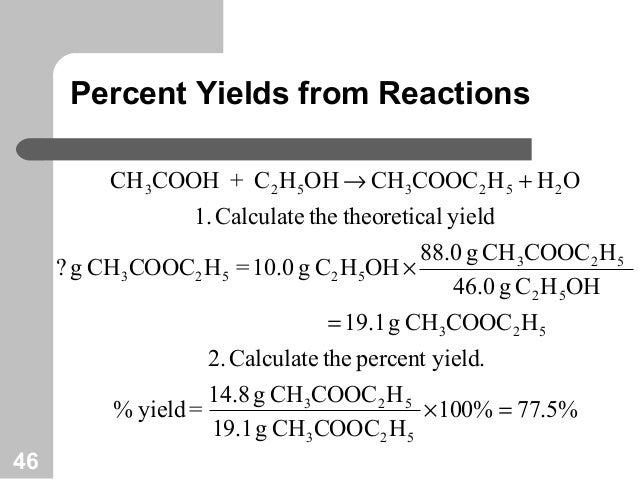


Chemical Equations And Reaction Stoichiometry



Calculating Percent Recovery Percent Yield
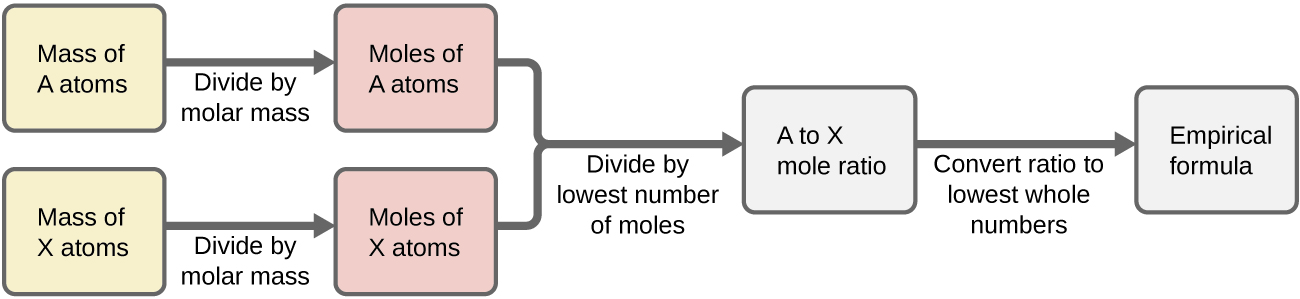


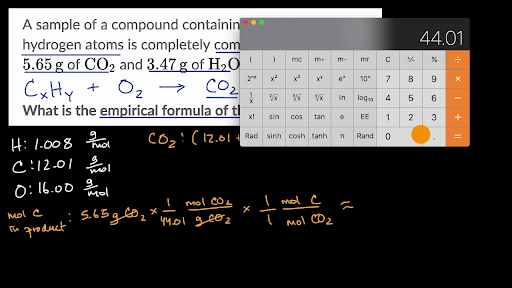
8 05 Empirical Formulas Chemistry Libretexts


Chapter 6 Summary Notes



Reaction Percent Yield Introduction And Practice Exercises



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Percent Yield Tutorial Explained Practice Problems Crash Chemistry Academy Youtube
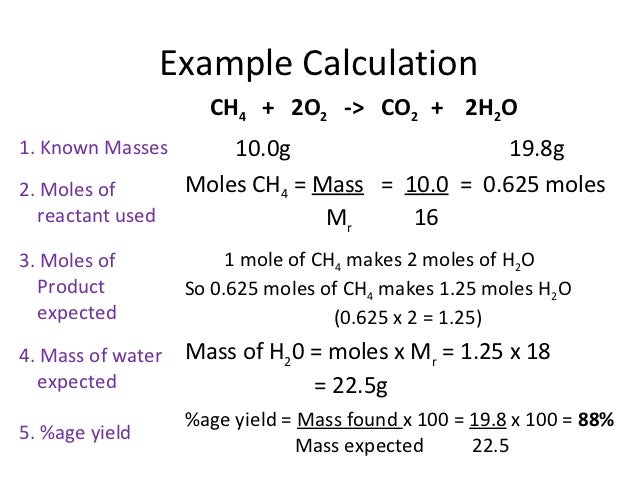


Chemistry Calculations Percent Yield And Atom Economy



Stoichiometry Percent Yield Stoichiometry Problems Clear Easy Youtube



Percentage Yield Lab Answers Schoolworkhelper



How To Calculate Theoretical Yield 12 Steps With Pictures
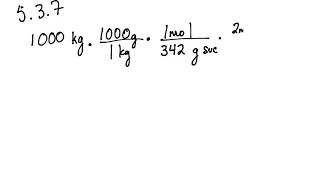


5 3 Calculating Reaction Yields Problems Chemistry Libretexts


Calculation Of Theoretical Yield Organic Chemistry I 212 01



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com
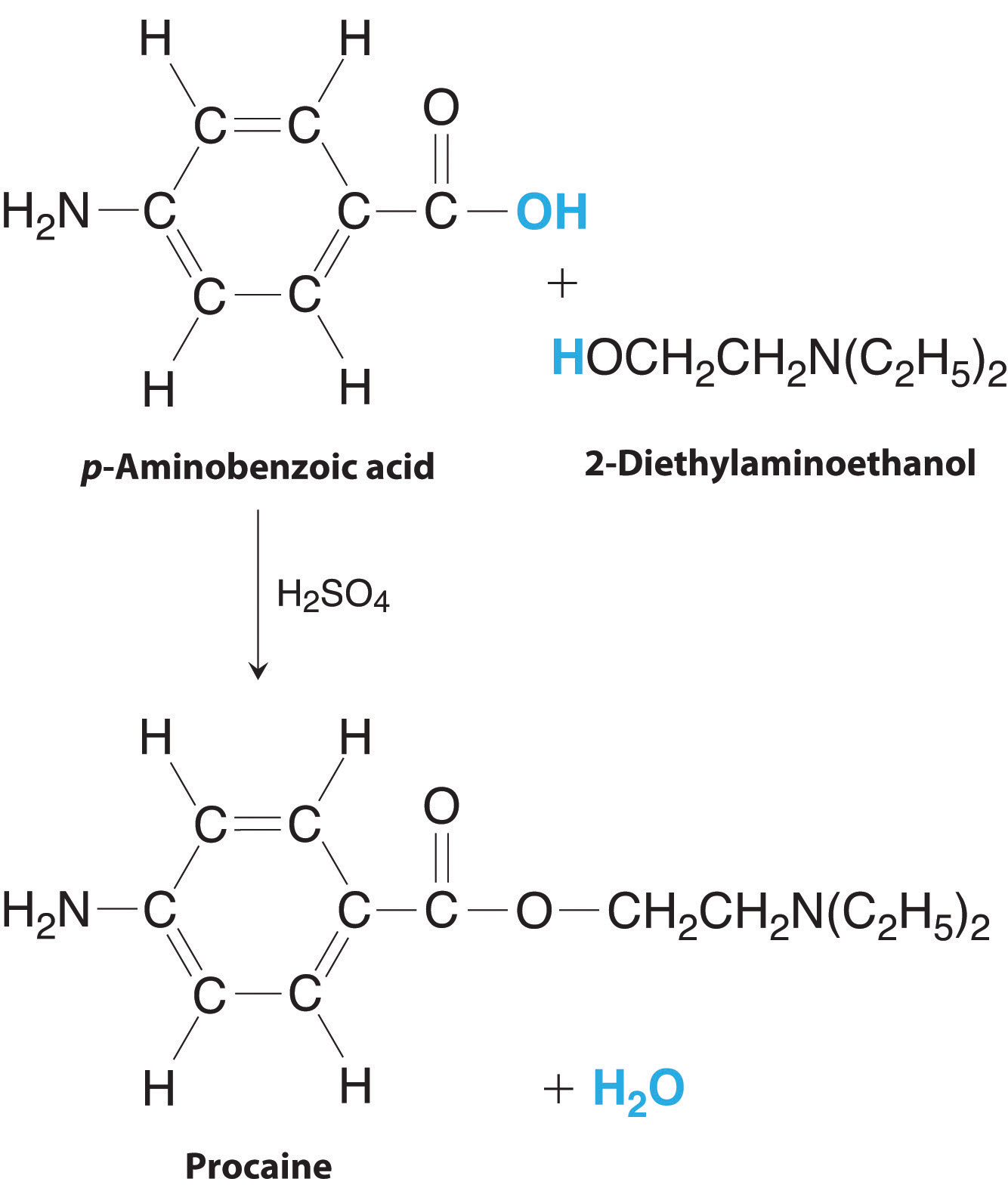


4 5 Other Practical Matters In Reactions Chemistry Libretexts
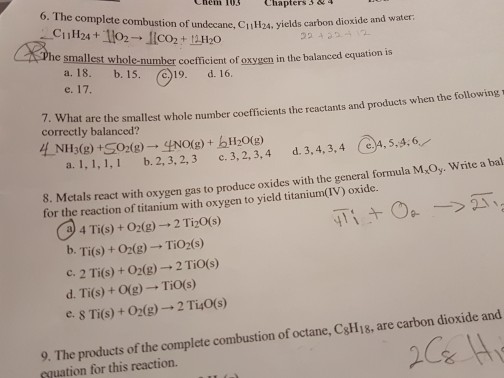


Solved The Complete Combustion Of Undecane C 11h 24 Yie Chegg Com


Secure Media Collegeboard Org Digitalservices Pdf Ap Apcentral Ap15 Chemistry Q2 Pdf



Percentage Yield Lab Answers Schoolworkhelper
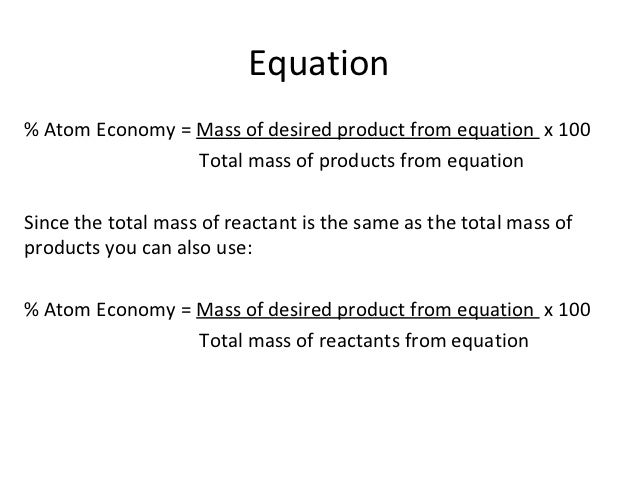


Chemistry Calculations Percent Yield And Atom Economy



Chemical Equations And Reaction Stoichiometry Ppt Download



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World
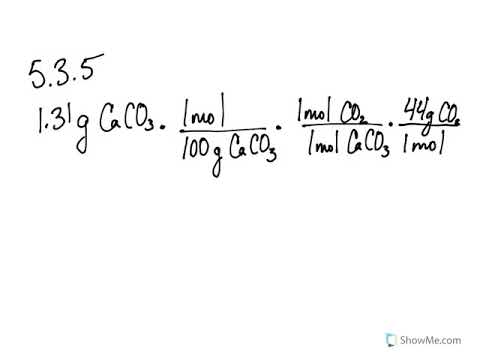


5 3 Calculating Reaction Yields Problems Chemistry Libretexts
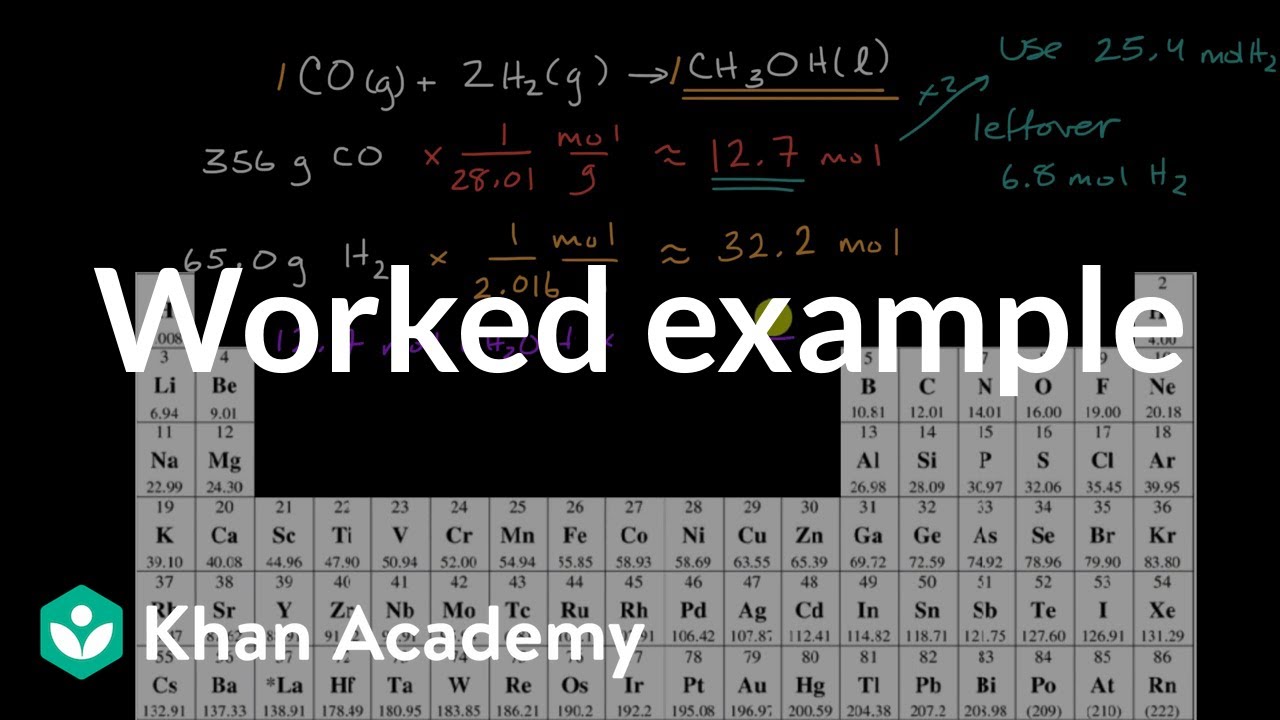


Calculating The Amount Of Product Formed From A Limiting Reactant Worked Example Video Khan Academy



Calculating Actual Yield Given The Percent Yield Youtube



Solved Lab Empirical Formula Yield Of Magnesium Oxide S Chegg Com



Limiting Reagent Stoichiometry Practice Khan Academy


Chemistry Atom Economy And Percentage Yield



Chemistry Calculations Percent Yield And Atom Economy



How To S Wiki How To Find Percentage Yield In Chemistry
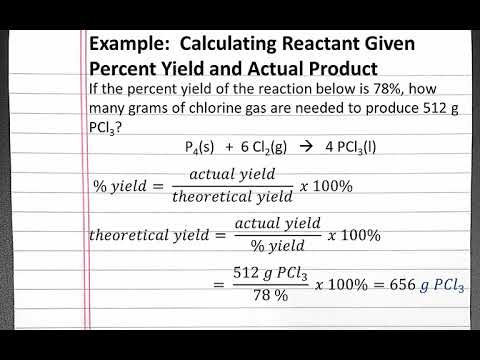


Chemistry 101 Calculating Reactant Given Percent Yield And Actual Product Youtube



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Yield Calc



Percentage Yield Lab Answers Schoolworkhelper



Calculating Percent Yield Hey Chemistry



Select A Section Introduction Stoichiometry Of Chemical Compounds 3 1 Molecular Masses And Formula Masses 3 2 The Mole And Avogadro S Number 3 3 The Mole And Molar Mass 3 4 Mass Percent Composition From Chemical Formulas 3 5 Chemical



Percent Yield Percent Purity Solutions Examples Videos


Exp6 Prepmethylbenzoate Chem234



Solved Awhsu Chem 48 5 Calculate The Mol Ratio Of Iodi Chegg Com



Yields Introductory Chemistry


Calculating Percentage Yield


Q Tbn And9gcs60s5akezigys4bjyqzve5tdrkwvatrchodzxvxw7 9iinr194 Usqp Cau



Calculating Percent Yield Hey Chemistry


The Stoichiometry Of Product Formation And Percent Yield
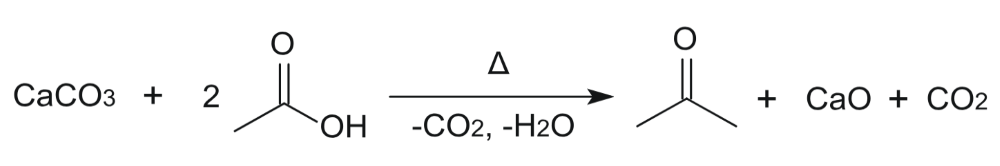


Theoretical Yield Calculator
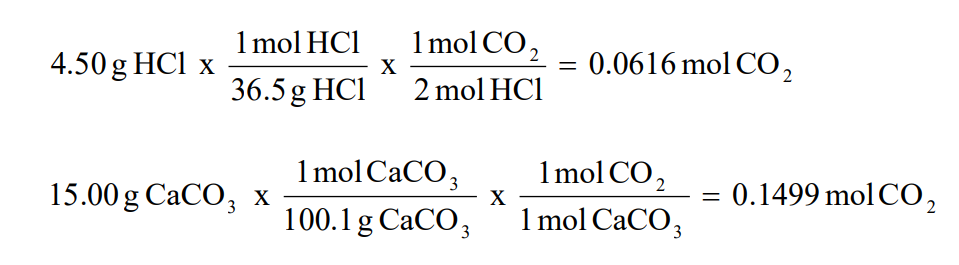


Molecular Formulas And Nomenclature



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com


How Do I Enter Chemistry Equations In Ms Word Libanswers



Multiple Reactions Yield Selectivity Youtube
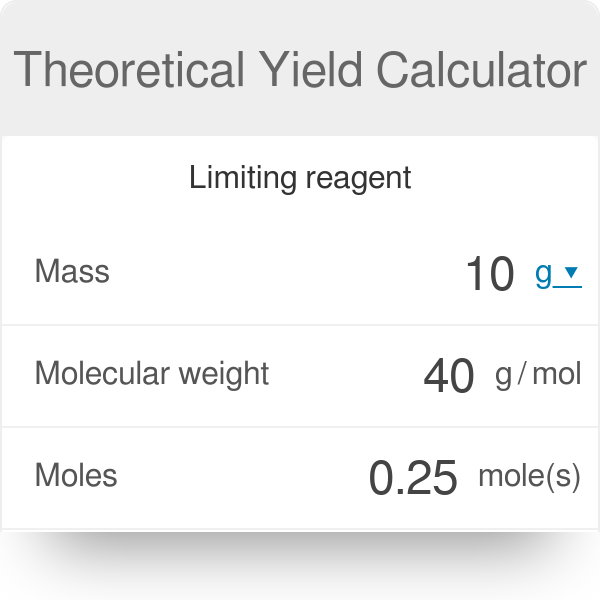


Theoretical Yield Calculator



How To Calculate Percent Yield In Chemistry 15 Steps



Chemical Reactions Chemistry For Non Majors



Howto How To Find Percent Yield Without Actual Yield



Ap Chemistry Unit 3
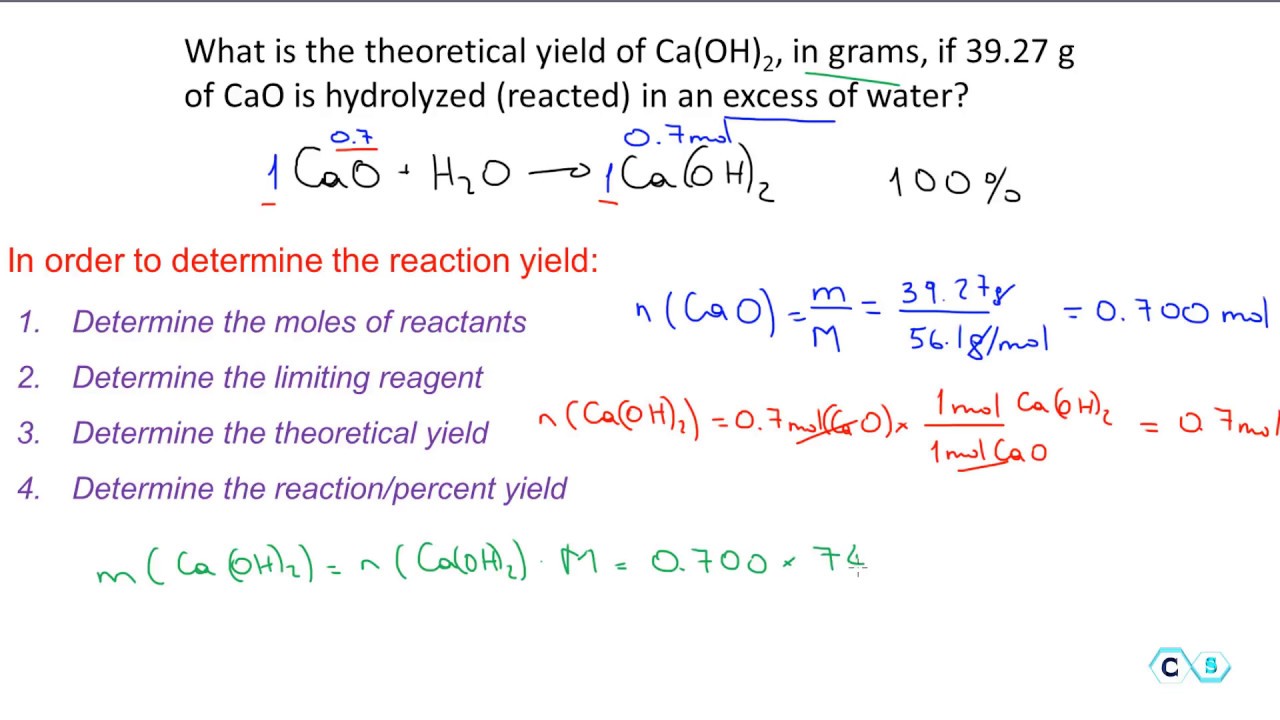


Reaction Percent Yield Introduction And Practice Exercises


8 4 Limiting Reactant And Theoretical Yield Chemistry Libretexts
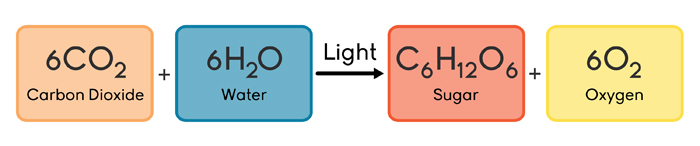


Quantifying Chemical Reactions Stoichiometry And Moles Annenberg Learner



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Using Evidence To Determine The Correct Chemical Equation A Stoichiometry Investigation Chemical Education Xchange



Yield Calculations Faculty Staff Sites
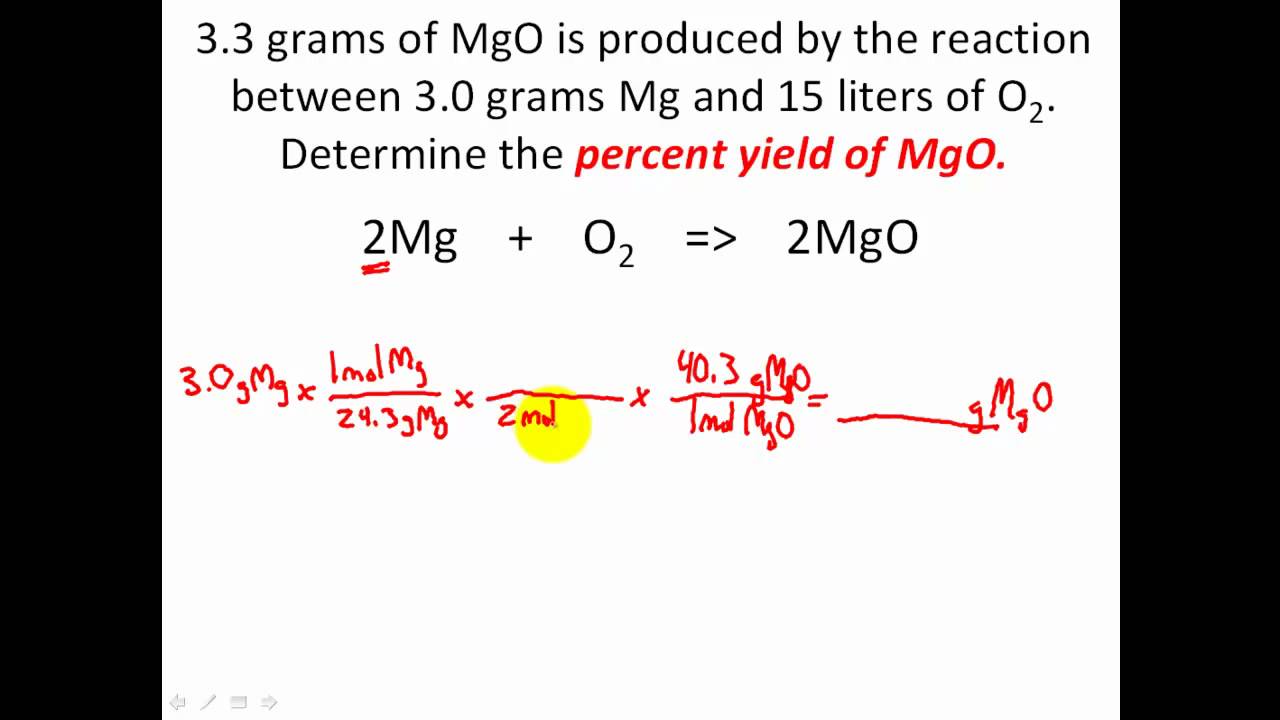


Stoichiometry Solving Percent Yield Stoichiometry Problems Youtube



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World



Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube
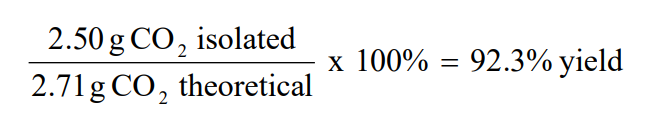


Molecular Formulas And Nomenclature



Reaction Yield An Overview Sciencedirect Topics
/148302528-56a12f323df78cf77268383a.jpg)


Percent Yield Definition And Formula



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Chemistry Atom Economy And Percentage Yield



Conversion Chemistry Wikipedia


コメント
コメントを投稿